How To Find Structural Formula In Chemistry
Atoms, Molecules, and Ions
Chemical Formulas
OpenStaxCollege
[latexpage]
Learning Objectives
By the end of this section, you volition exist able to:
- Symbolize the composition of molecules using molecular formulas and empirical formulas
- Represent the bonding system of atoms within molecules using structural formulas
A molecular formula is a representation of a molecule that uses chemic symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule. (A subscript is used only when more than than i atom of a given type is present.) Molecular formulas are too used as abbreviations for the names of compounds.
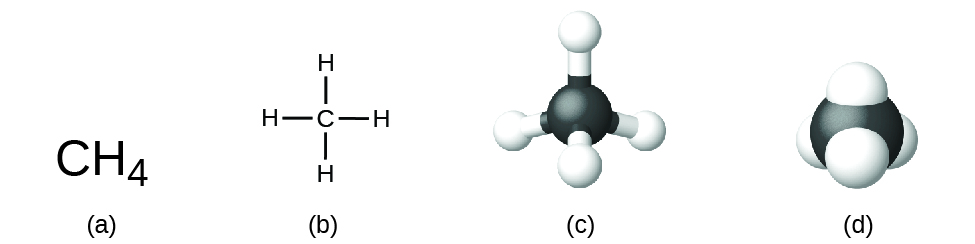
The structural formula for a compound gives the same information as its molecular formula (the types and numbers of atoms in the molecule) but also shows how the atoms are connected in the molecule. The structural formula for methyl hydride contains symbols for i C atom and four H atoms, indicating the number of atoms in the molecule ([link]). The lines stand for bonds that hold the atoms together. (A chemical bond is an attraction betwixt atoms or ions that holds them together in a molecule or a crystal.) We will discuss chemical bonds and see how to predict the arrangement of atoms in a molecule subsequently. For now, simply know that the lines are an indication of how the atoms are connected in a molecule. A ball-and-stick model shows the geometric arrangement of the atoms with atomic sizes not to scale, and a space-filling model shows the relative sizes of the atoms.
A methane molecule can be represented as (a) a molecular formula, (b) a structural formula, (c) a ball-and-stick model, and (d) a space-filling model. Carbon and hydrogen atoms are represented past blackness and white spheres, respectively.
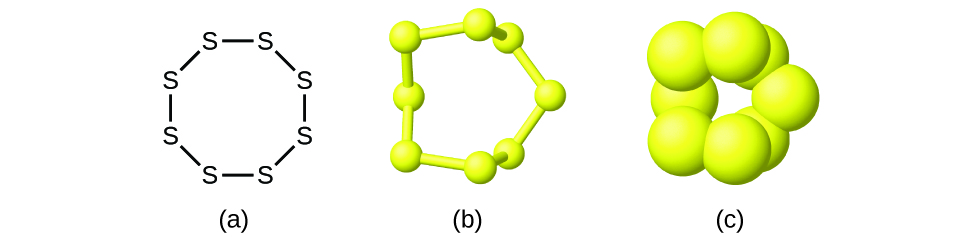
Although many elements consist of detached, private atoms, some exist as molecules made up of two or more atoms of the element chemically bonded together. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that comprise 2 atoms each (chosen diatomic molecules) and thus take the molecular formulas H2, O2, and Northtwo, respectively. Other elements commonly constitute as diatomic molecules are fluorine (Ftwo), chlorine (Cl2), bromine (Br2), and iodine (I2). The most common form of the chemical element sulfur is composed of molecules that consist of eight atoms of sulfur; its molecular formula is S8 ([link]).
A molecule of sulfur is equanimous of eight sulfur atoms and is therefore written as Sviii. It tin exist represented as (a) a structural formula, (b) a ball-and-stick model, and (c) a space-filling model. Sulfur atoms are represented by yellow spheres.
It is important to note that a subscript following a symbol and a number in front of a symbol do non represent the same matter; for case, Hii and 2H represent distinctly dissimilar species. H2 is a molecular formula; it represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together. The expression 2H, on the other hand, indicates 2 divide hydrogen atoms that are not combined equally a unit of measurement. The expression 2Htwo represents two molecules of diatomic hydrogen ([link]).
The symbols H, 2H, H2, and 2H2 represent very different entities.

Compounds are formed when two or more than elements chemically combine, resulting in the formation of bonds. For example, hydrogen and oxygen tin react to form water, and sodium and chlorine tin react to form table salt. We sometimes describe the limerick of these compounds with an empirical formula, which indicates the types of atoms present and the simplest whole-number ratio of the number of atoms (or ions) in the chemical compound. For example, titanium dioxide (used as pigment in white paint and in the thick, white, blocking type of sunscreen) has an empirical formula of TiO2. This identifies the elements titanium (Ti) and oxygen (O) as the constituents of titanium dioxide, and indicates the presence of twice as many atoms of the element oxygen as atoms of the element titanium ([link]).
(a) The white compound titanium dioxide provides constructive protection from the sun. (b) A crystal of titanium dioxide, TiO2, contains titanium and oxygen in a ratio of 1 to ii. The titanium atoms are grayness and the oxygen atoms are red. (credit a: modification of work by "osseous"/Flickr)

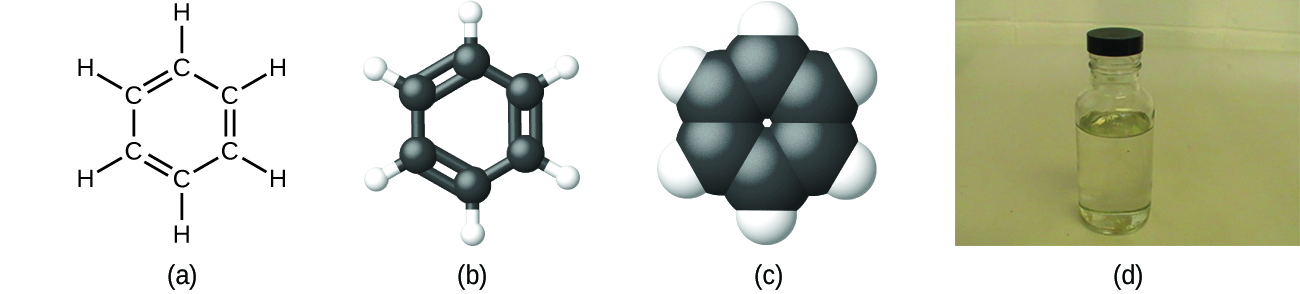
As discussed previously, we can describe a compound with a molecular formula, in which the subscripts indicate the actual numbers of atoms of each element in a molecule of the compound. In many cases, the molecular formula of a substance is derived from experimental determination of both its empirical formula and its molecular mass (the sum of atomic masses for all atoms composing the molecule). For case, it can exist determined experimentally that benzene contains two elements, carbon (C) and hydrogen (H), and that for every carbon atom in benzene, there is i hydrogen atom. Thus, the empirical formula is CH. An experimental determination of the molecular mass reveals that a molecule of benzene contains 6 carbon atoms and half dozen hydrogen atoms, then the molecular formula for benzene is CviH6 ([link]).
Benzene, C6Hsix, is produced during oil refining and has many industrial uses. A benzene molecule tin can be represented as (a) a structural formula, (b) a ball-and-stick model, and (c) a space-filling model. (d) Benzene is a clear liquid. (credit d: modification of work by Sahar Atwa)
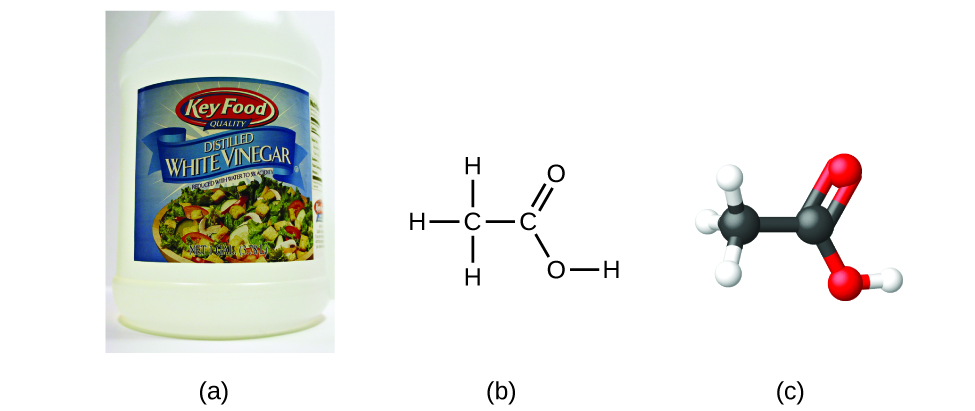
If we know a compound'southward formula, nosotros can easily determine the empirical formula. (This is somewhat of an bookish exercise; the opposite chronology is by and large followed in bodily practise.) For example, the molecular formula for acerb acid, the component that gives vinegar its precipitous taste, is CiiH4O2. This formula indicates that a molecule of acetic acid ([link]) contains two carbon atoms, four hydrogen atoms, and two oxygen atoms. The ratio of atoms is 2:4:2. Dividing by the lowest common denominator (2) gives the simplest, whole-number ratio of atoms, 1:2:1, so the empirical formula is CH2O. Note that a molecular formula is ever a whole-number multiple of an empirical formula.
(a) Vinegar contains acetic acid, C2HfourO2, which has an empirical formula of CH2O. It tin can be represented as (b) a structural formula and (c) as a brawl-and-stick model. (credit a: modification of piece of work by "HomeSpot HQ"/Flickr)
Empirical and Molecular Formulas
Molecules of glucose (claret saccharide) incorporate 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. What are the molecular and empirical formulas of glucose?
Solution
The molecular formula is C6H12O6 because one molecule really contains half dozen C, 12 H, and 6 O atoms. The simplest whole-number ratio of C to H to O atoms in glucose is one:two:1, so the empirical formula is CHtwoO.
Check Your Learning
A molecule of metaldehyde (a pesticide used for snails and slugs) contains 8 carbon atoms, xvi hydrogen atoms, and 4 oxygen atoms. What are the molecular and empirical formulas of metaldehyde?
Answer:
Molecular formula, CeightH16O4; empirical formula, C2HfourO
Lee Cronin
What is it that chemists do? Co-ordinate to Lee Cronin ([link]), chemists make very complicated molecules by "chopping up" small molecules and "reverse engineering science" them. He wonders if we could "make a really cool universal chemical science fix" past what he calls "app-ing" chemical science. Could nosotros "app" chemical science?
In a 2012 TED talk, Lee describes i fascinating possibility: combining a collection of chemical "inks" with a 3D printer capable of fabricating a reaction apparatus (tiny test tubes, beakers, and the similar) to fashion a "universal toolkit of chemistry." This toolkit could be used to create custom-tailored drugs to fight a new superbug or to "impress" medicine personally configured to your genetic makeup, environment, and health situation. Says Cronin, "What Apple did for music, I'd similar to do for the discovery and distribution of prescription drugs."one View his full talk at the TED website.
Pharmacist Lee Cronin has been named one of the UK's 10 most inspirational scientists. The youngest chair at the Academy of Glasgow, Lee runs a large inquiry group, collaborates with many scientists worldwide, has published over 250 papers in top scientific journals, and has given more than 150 invited talks. His research focuses on complex chemical systems and their potential to transform technology, but also branches into nanoscience, solar fuels, synthetic biology, and fifty-fifty artificial life and evolution. (credit: image courtesy of Lee Cronin)

Information technology is important to be aware that it may exist possible for the same atoms to be arranged in different ways: Compounds with the same molecular formula may have unlike atom-to-atom bonding and therefore different structures. For case, could there be some other compound with the same formula as acetic acrid, C2H4Otwo? And if so, what would be the structure of its molecules?
If you predict that another compound with the formula C2H4O2 could exist, then you demonstrated good chemical insight and are correct. Two C atoms, 4 H atoms, and two O atoms can as well be arranged to course a methyl formate, which is used in manufacturing, as an insecticide, and for quick-drying finishes. Methyl formate molecules have ane of the oxygen atoms betwixt the 2 carbon atoms, differing from the arrangement in acetic acid molecules. Acetic acrid and methyl formate are examples of isomers—compounds with the aforementioned chemical formula but different molecular structures ([link]). Note that this small difference in the system of the atoms has a major effect on their corresponding chemic properties. You would certainly not want to use a solution of methyl formate every bit a substitute for a solution of acetic acrid (vinegar) when yous make salad dressing.
Molecules of (a) acerb acid and methyl formate (b) are structural isomers; they have the aforementioned formula (C2H4Otwo) merely different structures (and therefore dissimilar chemic backdrop).

Many types of isomers exist ([link]). Acetic acid and methyl formate are structural isomers, compounds in which the molecules differ in how the atoms are continued to each other. There are likewise various types of spatial isomers, in which the relative orientations of the atoms in infinite tin be different. For example, the compound carvone (constitute in caraway seeds, spearmint, and mandarin orange peels) consists of two isomers that are mirror images of each other. S-(+)-carvone smells like caraway, and R-(−)-carvone smells similar spearmint.
Molecules of carvone are spatial isomers; they only differ in the relative orientations of the atoms in infinite. (credit bottom left: modification of work by "Miansari66"/Wikimedia Commons; credit bottom right: modification of work by Forest & Kim Starr)
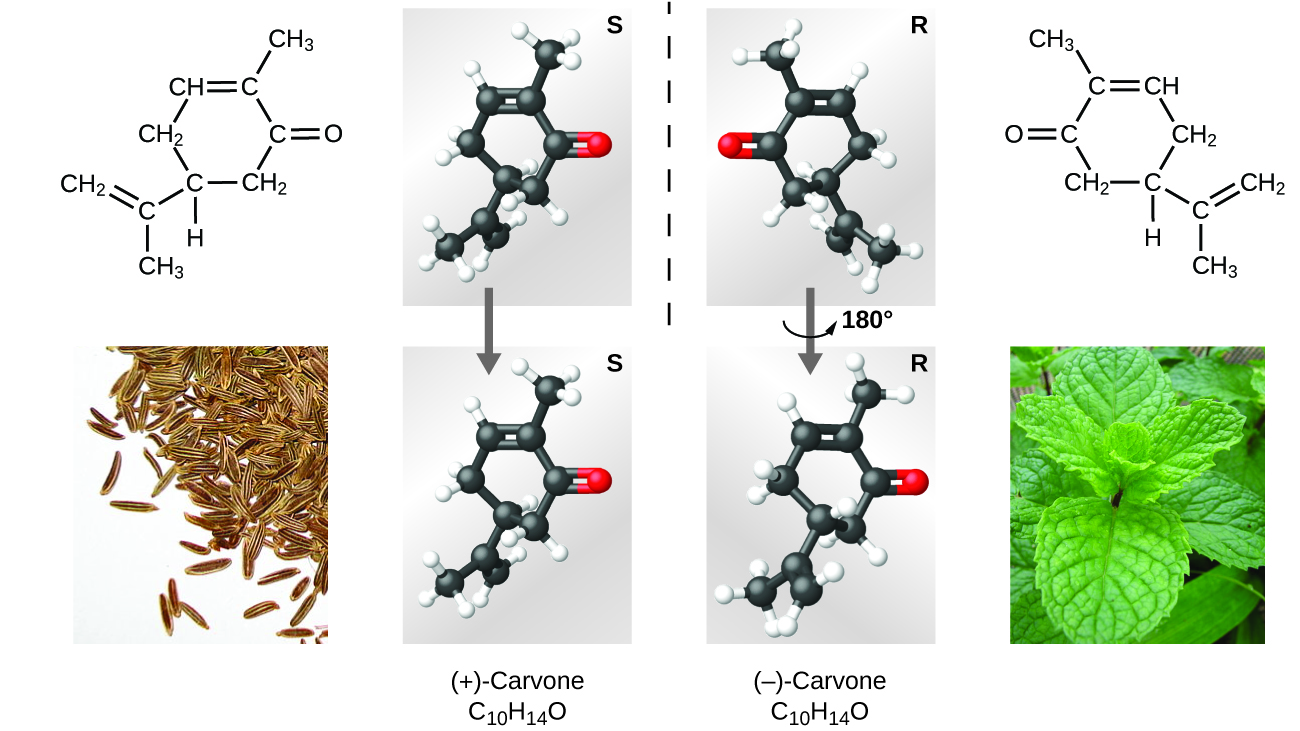

Select this link to view an explanation of isomers, spatial isomers, and why they have unlike smells (select the video titled "Mirror Molecule: Carvone").
Key Concepts and Summary
A molecular formula uses chemical symbols and subscripts to indicate the verbal numbers of different atoms in a molecule or compound. An empirical formula gives the simplest, whole-number ratio of atoms in a compound. A structural formula indicates the bonding arrangement of the atoms in the molecule. Ball-and-stick and space-filling models show the geometric arrangement of atoms in a molecule. Isomers are compounds with the aforementioned molecular formula just unlike arrangements of atoms.
Chemical science Terminate of Chapter Exercises
Explain why the symbol for an atom of the element oxygen and the formula for a molecule of oxygen differ.
The symbol for the chemical element oxygen, O, represents both the element and one atom of oxygen. A molecule of oxygen, Otwo, contains two oxygen atoms; the subscript 2 in the formula must be used to distinguish the diatomic molecule from two unmarried oxygen atoms.
Explain why the symbol for the element sulfur and the formula for a molecule of sulfur differ.
Write the molecular and empirical formulas of the following compounds:
(a)
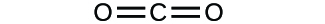
(b)
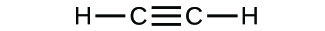
(c)

(d)
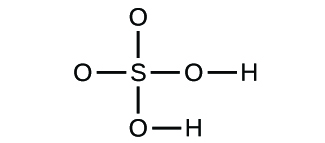
(a) molecular COii, empirical COii; (b) molecular C2H2, empirical CH; (c) molecular C2Hfour, empirical CH2; (d) molecular H2SOfour, empirical H2So4
Write the molecular and empirical formulas of the post-obit compounds:
(a)
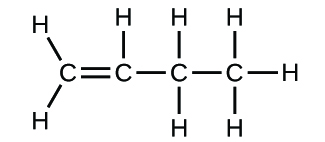
(b)
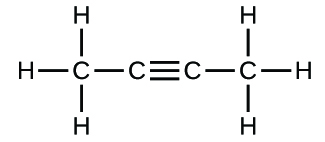
(c)
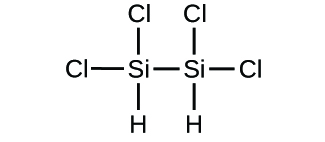
(d)
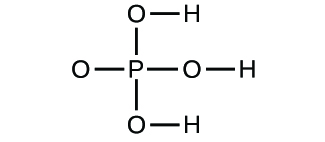
Determine the empirical formulas for the post-obit compounds:
(a) caffeine, C8HxN4O2
(b) fructose, C12H22O11
(c) hydrogen peroxide, H2Otwo
(d) glucose, C6H12O6
(e) ascorbic acrid (vitamin C), C6H8O6
(a) CivH5N2O; (b) C12H22O11; (c) HO; (d) CHtwoO; (due east) CthreeH4O3
Determine the empirical formulas for the following compounds:
(a) acerb acrid, CtwoH4Oii
(b) citric acid, C6H8O7
(c) hydrazine, NtwoH4
(d) nicotine, CtenH14N2
(eastward) butane, C4H10
Write the empirical formulas for the post-obit compounds:
(a)
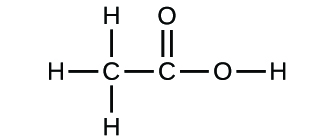
(b)
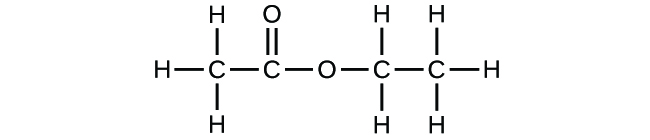
(a) CH2O; (b) C2HfourO
Open the Build a Molecule simulation and select the "Larger Molecules" tab. Select an appropriate atoms "Kit" to build a molecule with 2 carbon and six hydrogen atoms. Drag atoms into the space above the "Kit" to make a molecule. A proper name will announced when you take made an bodily molecule that exists (even if it is not the one you want). You tin employ the scissors tool to carve up atoms if yous would similar to change the connections. Click on "3D" to see the molecule, and look at both the space-filling and ball-and-stick possibilities.
(a) Draw the structural formula of this molecule and state its name.
(b) Can y'all accommodate these atoms in any way to make a different compound?
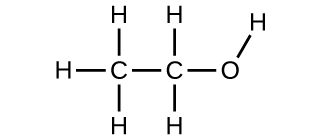
Utilise the Build a Molecule simulation to echo [link], just build a molecule with two carbons, 6 hydrogens, and one oxygen.
(a) Draw the structural formula of this molecule and state its name.
(b) Can you adjust these atoms to make a dissimilar molecule? If so, draw its structural formula and state its proper noun.
(c) How are the molecules fatigued in (a) and (b) the same? How do they differ? What are they chosen (the type of relationship between these molecules, not their names).
(a) ethanol
(b) methoxymethane, more than ordinarily known equally dimethyl ether
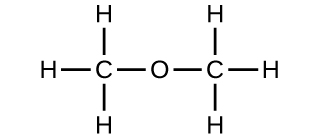
(c) These molecules take the same chemic composition (types and number of atoms) just different chemical structures. They are structural isomers.
Apply the Build a Molecule simulation to repeat [link], simply build a molecule with three carbons, seven hydrogens, and one chlorine.
(a) Draw the structural formula of this molecule and state its proper noun.
(b) Tin can you conform these atoms to make a different molecule? If so, draw its structural formula and state its name.
(c) How are the molecules fatigued in (a) and (b) the same? How do they differ? What are they called (the type of relationship betwixt these molecules, not their names)?
Footnotes
- one Lee Cronin, "Impress Your Own Medicine," Talk presented at TED Global 2012, Edinburgh, Scotland, June 2012.
Glossary
- empirical formula
- formula showing the composition of a chemical compound given equally the simplest whole-number ratio of atoms
- isomers
- compounds with the same chemic formula simply different structures
- molecular formula
- formula indicating the limerick of a molecule of a compound and giving the actual number of atoms of each element in a molecule of the compound.
- spatial isomers
- compounds in which the relative orientations of the atoms in space differ
- structural formula
- shows the atoms in a molecule and how they are connected
- structural isomer
- i of two substances that have the same molecular formula only unlike physical and chemic properties because their atoms are bonded differently
How To Find Structural Formula In Chemistry,
Source: https://pressbooks-dev.oer.hawaii.edu/chemistry/chapter/chemical-formulas/
Posted by: losoyawhavuld.blogspot.com


0 Response to "How To Find Structural Formula In Chemistry"
Post a Comment